Safe Blood Transport in Hospitals: Best Practices for Carriers, Labeling, and Routes
Every day, hospitals move hundreds of blood samples and blood products between wards, emergency departments, operating rooms, and the lab.
Most of those journeys are uneventful. But when something goes wrong (mislabeling, leakage, hemolysis, or a delayed delivery), the stakes are high: delayed treatment, repeat collections, transfusion risks, and potential harm to patients.
Safe blood transportation is not only a logistics question. It's a core element of patient safety and laboratory quality.
This guide walks through practical best practices for labeling and identification, carriers and containers for blood, route selection and transport methods, and policies for when to use (and not use) pneumatic tubes, so that operations leaders, lab managers, and clinical teams can align on a safe, efficient approach.
Why Blood Transport Needs Special Attention
Blood specimens and blood products are different from many other items hospitals move:
They are directly tied to critical decisions (diagnosis, transfusion, medication dosing).
They can degrade or be altered by time, temperature, and mechanical stress.
They fall under strict regulatory, accreditation, and safety requirements.
Even when overall laboratory processes are strong, weak points in blood delivery and handling can lead to:
Increased hemolysis and rejected samples
Recollection and patient discomfort
Lost units or misdirected products
Delayed or inappropriate transfusions
Getting the basics right is essential.
Best Practices for Labeling and Identification
Correct labeling is the foundation of safe blood transport. No amount of carrier sophistication can fix a mislabeled tube.
Positive Patient Identification at Collection
At draw time:
Use at least two unique identifiers (full name, date of birth, MRN).
Follow institutional policies for patient confirmation (wristband scanning, verbal confirmation, etc.).
Avoid pre-labeling tubes before collection whenever possible.
For transfusion-related testing, many hospitals require enhanced identification steps (such as second signatures or independent verification). These must be followed rigorously before specimens even enter the transport workflow.
Bedside Labeling with Barcodes
The right approach:
Print labels at the bedside or clinic station directly from the LIS/EHR order.
Attach labels immediately after collection and verification.
Use barcodes that encode key data (patient, test, collection time).
This supports:
Accurate tracking from collection through blood transportation
Fewer relabeling steps in transit
Easier integration with tube station scanners and lab accessioning
Clear Routing and Priority Labels
When specimens or blood products are placed into carriers or transport bags:
Use standardized stickers or fields to indicate destination ("Core Lab," "Blood Bank," "ICU 4C") and priority (STAT, urgent, routine).
Avoid handwritten, ad-hoc notes where possible. They are easily misread or missed.
For blood products, include or attach transfusion requisitions as per policy.
Consistent visual cues reduce routing errors and speed up recognition at receiving points.
Choosing the Right Containers and Carriers for Blood
Safe blood transport depends heavily on what you put samples in, not only how you move them.
Primary Containers: Blood Tubes and Bags
For blood samples:
Use appropriate tube types (serum, heparin, EDTA, citrate, etc.) for ordered tests.
Follow correct order of draw to minimize additive carryover.
Make sure tubes are filled to the correct volume (especially for coagulation tests).
For blood products, follow blood bank and manufacturer guidelines for handling, orientation, and allowable vibration/temperature ranges.
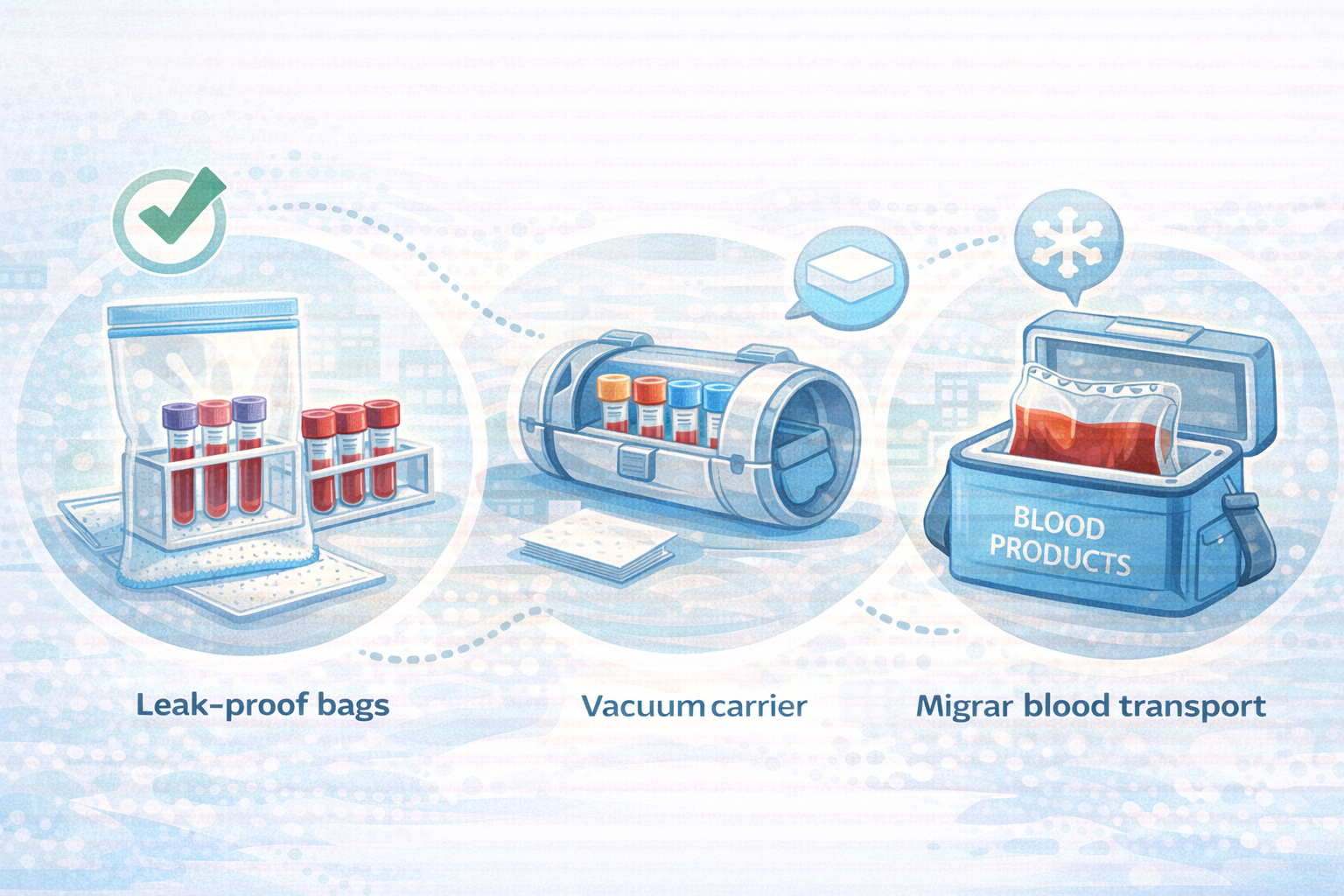
Secondary Containers: Racks, Bags, and Carriers
Regardless of transport method, use a secondary containment layer:
Leak-proof bags or containers to prevent spills
Rigid racks or inserts to keep tubes upright and separated
Absorbent material where policies require it
For pneumatic tube transport, carriers effectively become the secondary container. For manual transport, this might be a rigid box or insulated container.
Carriers for Pneumatic Tube Systems
For hospitals using a pneumatic tube system hospital-wide, proper carrier setup is critical:
Use carriers with intact seals, latches, and padding.
Insert foam or custom-fit holders designed for blood tubes where available.
Avoid overloading carriers. Follow manufacturer recommendations for maximum tube count and weight.
Overloaded or poorly cushioned carriers increase mechanical stress on samples and can contribute to hemolysis or broken tubes.
Routes and Methods: When to Walk, When to Tube
Different transport options have different risk and performance profiles.
Manual Hand-Carry
Works best for:
High-risk or restricted items (certain blood products where tube use has not been validated)
Situations where tube systems are down or routes are unavailable
Very short distances when speed and control are both high
What works:
Use designated, trained staff where possible (porters or lab couriers).
Use rigid, closed containers, not open racks, when moving through public spaces.
Follow clear handoff rules (who is responsible at each step).
Scheduled Courier or Porter Routes
Often used for:
Inter-building transfers
Outpatient clinics or satellites
Night shift or low-volume periods
What works:
Align schedules with known peak draw times to avoid long waits.
Use appropriate insulated containers when temperature control matters.
Maintain logs for critical blood product transfers as required by regulation.
Pneumatic Tube Transport
In hospitals with validated systems, pneumatic tubes can safely move many blood samples and, in some organizations, selected blood products.
What works (building on your validation work):
Only send specimen types that are explicitly allowed by your validated policy.
Use proper carriers and inserts, as described above.
Follow station-level SOPs for packaging, labeling, and dispatch.
Defining a Clear Blood Transport Policy
Safe practice requires more than informal habits. Hospitals need a written, shared blood transport policy that answers:
Which blood samples can go via pneumatic tube?
Which blood products, if any, can go via tube, and under what conditions?
Which items must always be hand-carried?
Who is responsible for packaging, dispatch, and receipt at each step?
Acceptable Specimens and Products
Work with lab leadership and blood bank, transfusion medicine specialists, and quality and risk management to define:
A list of acceptable specimens to transport via pneumatic tube (routine chem/hematology, certain coag tests).
A list of items that must not be sent by tube (or that require extra preparation, like double-bagging or specialized inserts).
These lists should be published in SOPs and transport guides, available at collection points and tube stations, and incorporated into training for new staff.
Priority and Routing Rules
For blood samples, define when "STAT" truly applies (ED critical labs, OR intra-op tests). Use tube system priority features so STAT samples bypass routine traffic where supported.
For blood products, clearly define target delivery times from blood bank to unit or OR. Specify when dedicated couriers vs porters vs tube systems are used, based on risk and validation.
Operational Tips to Reduce Risk in Daily Blood Transport
Even with good policies, day-to-day practices can make or break safety.
Make Stations and Handoffs Obvious
Clearly label hospital tube stations with destinations, acceptable item lists, and emergency contact info. Use visual aids (laminated cards, posters) showing correct packaging for blood samples. Standardize how specimens are placed in carriers or containers across units.
Train, Retrain, and Refresh
Include blood transport in nurse and phlebotomist onboarding, annual competency assessments, and unit-based safety huddles and refreshers.
Focus training on:
Labeling and ID
What can and cannot be sent by tube
Proper packaging in carriers and containers
What to do in case of spills, breakage, or delays
Monitor and Respond to Signals
Track and periodically review:
Hemolysis and sample rejection rates by unit and transport method
Incidents of misdirected or delayed blood delivery
Near-misses and reported concerns from staff
Use this data to adjust training focus, revise policies or routing, and fine-tune pneumatic tube routes and speeds if needed.
Atreo's Role in Safer Blood Transport
Atreo's work with hospital logistics goes beyond moving "things" quickly. It includes protecting pre-analytical quality and patient safety.
For blood transport, that looks like:
Designing and tuning tube networks with lab and blood bank input
Supporting hospitals in validating tube routes specifically for blood samples and, where appropriate, blood products
Helping translate validation data into simple, usable policies at the tube station and ward level
The goal: ensure that when blood travels (whether in a blood tube inside a carrier or in a transfusion bag in a container), its journey is as safe and predictable as possible.
Key Takeaways for Safe Blood Transport
Safe blood transport is about labeling, containers, and routes, not one technology or department alone.
Positive patient ID and bedside/barcode labeling are non-negotiables for safe blood sample collection and movement.
Use appropriate containers and carriers for blood, with secondary containment and proper cushioning, especially for tube systems.
Define clear, validated policies for what can go through a pneumatic tube system hospital-wide and what must be hand-carried.
Continuous monitoring and staff training keep daily practice aligned with policy and protect patients from avoidable harm.
Next Step: Schedule a Support Consultation on Pre-Analytical Validation
If your team has ever hesitated about sending blood via tube, or regularly deals with hemolysis, rejected samples, or delayed blood product deliveries, a focused review can help.
Schedule a Support Consultation
We'll:
Review your current blood transport policies for samples and products
Assess carriers, containers, and tube routes used for blood items
Recommend targeted improvements in packaging, routing, and training to reduce risk
Frequently Asked Questions About Blood Transport in Hospitals
Can we send blood products through a pneumatic tube system?
It depends on your specific system and validation data. Some hospitals successfully transport selected blood products via tube after thorough validation, while others restrict blood products to manual courier. Decisions should be based on local evidence and transfusion medicine policies, not assumptions.
How do we reduce hemolysis linked to blood transport?
Focus on proper collection technique and tube selection, using carriers with appropriate inserts and not overloading them, validating tube routes and adjusting speed/pressure profiles where needed, and monitoring hemolysis rates by route and unit, then acting on trends.
Who "owns" blood transport safety?
It's shared. Lab and blood bank teams, nursing, operations, and whoever manages your transport infrastructure (porters, courier services, tube system teams) all play a role. The safest systems are those where responsibilities are clearly defined and everyone understands how their part protects patients.